Research
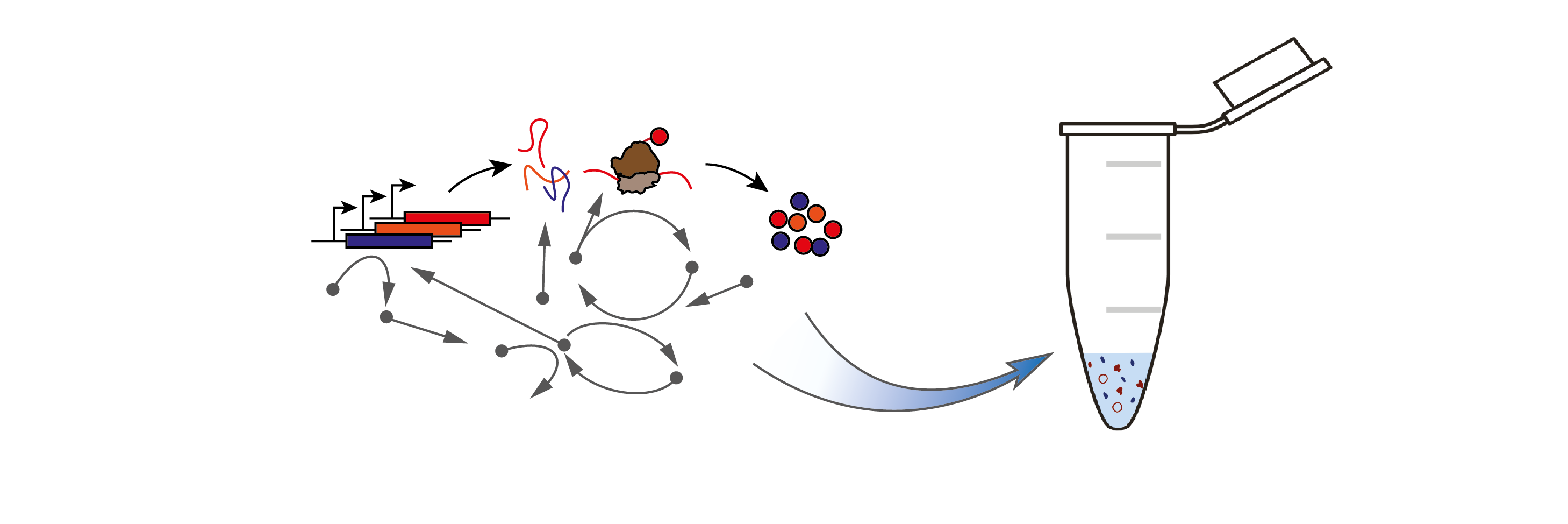
Synthetic biology is a rapidly growing field set to make profound impact in numerous industries including manufacturing, healthcare, agriculture, and sustainable energy, as well as our fundamental understanding of life itself. However, engineering synthetic biological systems reliably has proven to be a tremendously challenging task.
One promising approach is to use cell-free gene expression systems. These are in vitro systems that mimic the cellular environment and can be integrated with microfluidic technologies to rapidly prototype and screen synthetic gene circuits, prior to their deployment in vivo. This speeds up the synthetic biology design cycle, but also perhaps more powerfully, releases gene expression from the confines of the cell. In this way, the cell-free extracts themselves can be viewed as functional aqueous solutions, programmed by DNA.
The aim of our research is to harness these in vitro biomolecular mechanisms in a controlled and quantitative manner.
The consequence of this approach is twofold: the first is a deeper understanding of life, as we attempt to control and manipulate genetic and metabolic systems which have been crafted over billions of years of evolution. The second is the development of powerful cell-free applications which, unlike synthetic cellular systems, are not limited by the strict requirements to maintain life.
We currently have projects spanning four themes:
Optimisation - Cell-free protein synthesis (CFPS) is a complex biochemical reaction, and optimising it to produce specific proteins with high titres and functionality is not a trivial task. We are designing automated pipelines to combine data, models, liquid handling (using low-cost Opentrons machines as well as the full resources of the Edinburgh Genome Foundry), and statistical techniques to improve CFPS performance for specific proteins, by modulating reaction composition as well as judiciously supplementing the reaction with protein and small-molecule factors. People involved: Nicoll Zeballos Lema, Sahan Liyanagedera
Metabolism - Decades of studies have highlighted the key effects that the residual background metabolism of the lysate has on CFPS, and major improvements to reaction performance have been achieved by modulating this metabolism. We are seeking to understand the principles that govern cell-free metabolism, particularly in terms of energy and cofactor regeneration, by carefully analysing the metabolomics profiles of CFPS reactions, as well as constructing and testing our own synthetic pathways. People involved: Tony Bougas, Aran Purdy
Microfluidics - Combining CFPS with microfluidic devices has enabled studies at high throughput, and with exquisite control. We are using our high-throughput microfluidic platform to carry out enzyme engineering, and microfluidic chemostats to investigate the properties of CFPS reactions that regenerate their own components, as a precursor to replicative synthetic cells. People involved: Christoph Wagner, Sarah Paterson
Modelling - In-silico models of CFPS can guide fundamental understanding, optimisation efforts, and reduce experimental costs, by directing us to more informative measurements. We are developing mechanistic, predictive models of CFPS, and applying them to inference and experimental design problems. People involved: Christoph Wagner
Funding
We gratefully acknowledge support from the following organizations:






